Needle-free vial access cap
Needle-free vial access cap without Bionector
Description
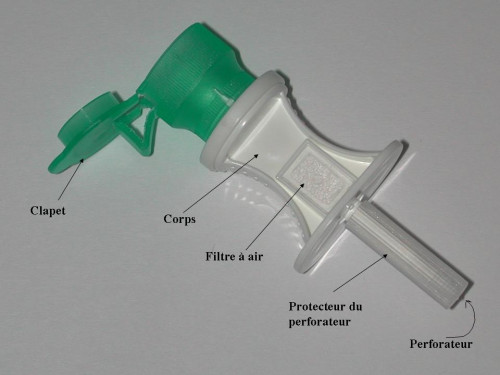
Single use, sterile, needleless device intended for fluid withdrawal, powdered medicine reconstitution and injection. It is recommended for the… Read more
Indication
It is recommended for handling standard products in vials under optimal aseptic conditions.
Description
Single use, sterile, needleless device intended for fluid withdrawal, powdered medicine reconstitution and injection.It is recommended for the reconstitution of sterile medicines: the preparation of powdered or lyophilisate solutions, preparation of eye drops, etc, and for repeated withdrawals from the same vial using a syringe.
References and Features
| Spike | Packaging | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Favourites | Code | Filter porosity µm | Filtering area cm² | Flowrate ml/min | Priming volume ml | Utilization pressure (maximum) bar | Length mm | Int. Ø mm | Ext. Ø mm | Units/Box | Units/Case |
| 281.01 | 0.1 | 0.36 | 39 à 12 mbar | 0.16 | 1 | - | - | - | 300 | 300 | |
| 281.02 | 5 | 1.29 | - | 0.16 | 1 | - | - | - | 300 | 300 | |
| 281.062 | - | - | - | 0.54 | - | 31.5 | 2.50 | 3.70 | 10 | 240 | |
Additional information
-
Contains Latex No
-
Contains animal product No
-
Pyrogen-free No