Neonatology: issues and precautions in enteral feeding of preterm babies
Because of their physiological immaturity, preterm babies have specific needs, which means they require medical devices adapted to specific administration volumes. Enteral nutrition is a crucial but also complex challenge in neonatal care.
Because of their physiological immaturity, preterm babies have specific needs, which means they require medical devices adapted to specific administration volumes. Enteral nutrition is a crucial but also complex challenge in neonatal care.
Neonatology: issues and precautions in enteral feeding of preterm babies
Because of their physiological immaturity, preterm babies have specific needs, which means they require medical devices adapted to specific administration volumes. Enteral nutrition is a crucial but also complex challenge in neonatal care.
The insertion and use of gastric tubes is one of the most common medical procedures in the neonatal intensive care unit. These tubes are used for gastric decompression, enteral nutrition and drug administration(1).
Let's take a look at the risks involved and the best practices to be aware of in order to optimise their use.
(1)Tamara Wallace, DNP, APRN, NNP-BC; Deborah Steward, PhD, RN (2014) Gastric Tube Use and Care in the NICU, NAINR. 2014;14(3):103-108.

Enteral nutrition for preterm babies: risks to be aware of
When it comes to enteral nutrition, managing the risk of connection errors with medical devices requires a holistic approach that takes into account the design and compliance of the medical devices, the training of healthcare staff, standard operating procedures and communication between the various members of the medical team. Guaranteeing and maintaining the health of infants requires the monitoring of:
-
Calculations and dosage:
Errors in drug dosage are potentially dangerous for preterm patients, and can lead to complications such as respiratory distress, cardiovascular problems and increased drowsiness when opioids are administered, for example.
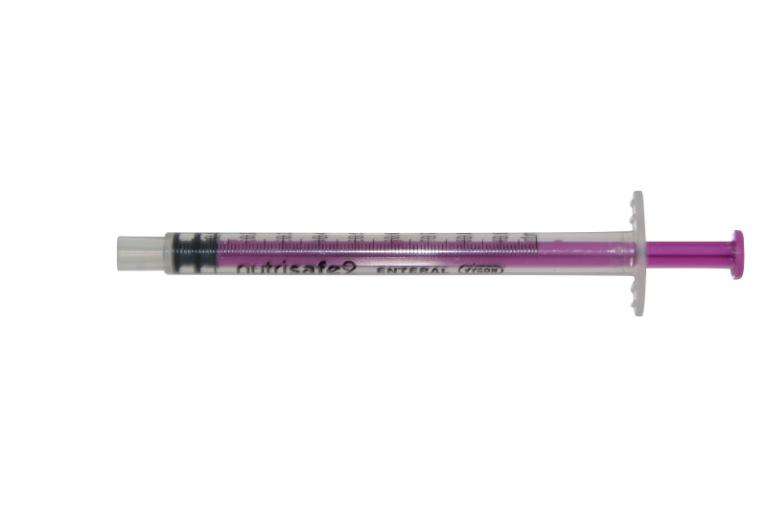
It is therefore necessary to use a system specially designed for neonatology.
“The volume of the displacement when making a connection also matters. In a 500g newborn infant, enteral drugs are often prescribed in volumes as small as 0.1 ml or even 0.01 ml” (2). As a result, a large-volume displacement, as indicated in ISO 80369-3 and which triggers an alert on the ENFitTM connector, cannot be tolerated. (2)
(2) Extract of ISO 80369-3 - Annex E - §E.5 Generic user needs
-
Is the accuracy of ENFit™ connectors suitable for newborn babies?
-
Laboratory testing shows that a mid-tolerance ENFit™ connector pair in a female-to-male orientation displaces an average volume of 0.148 ml of fluid, i.e., over-dosing of 0.148 ml (3)
(3) Extract of ISO 80369-3 - Annex A - Subpopulation within the enteral clinical application
On 12 October 2021, the FDA published a safety communication on LDT syringes relating to a risk of drug over-dosing with the ENFit® syringe with low-dose tip, based on a simulation carried out by ANDHEO, a specialised independent company, revealing over-dosing of 0.120 ml.
(4) Summary report - Study on the over-dosing risk of the "ENFit® Low Dose Tip" syringe during its use - March 2018, ANDHEO & Vygon.
- Journal of Clinical Pharmacy and Therapeutics - 2018(1)
This study raises clinical concerns about dosing inaccuracies with LDT syringes, particularly with the 0.5 and 1 ml syringes.
(1) O'Mara K, Gattoline SJ, Campbell CT. Female low dose tip syringes-increased complexity of use may compromise dosing accuracy in paediatric patients. J Clin Pharm Ther. 2019 Jun;44(3):463-470. doi: 10.1111/jcpt.12810. Epub 2019 Feb 14. PMID: 30763471 - Journal of Clinical Pharmacy and Therapeutics - 2019(2)
The use of oral adapters was also associated with an increased risk of inaccurate dosing.
Enteral and oral applications of LDT syringes produce unacceptable levels of dosage variance for high-risk drugs with a narrow therapeutic index.
(2) O'Mara K, Campbell C. Dosing inaccuracy with enteral use of ENFit ® low-dose tip syringes: The risk beyond oral adapters. J Clin Pharm Ther. 2020 Apr;45(2):335-339. doi: 10.1111/jcpt.13079. Epub 2019 Nov 22. PMID: 31755574. - Journal of Pediatric Pharmacology and Therapeutics - 2023(3)
This study confirms that ENFit® LDT syringes present a higher risk of dosing inaccuracy than other available models, even when used with bulk vial adapters.
(3) O’Mara K, Campbell C, O’Mara R. Comparison of Dosing Accuracy Between the ENFit LDT and a Neonatal-Specifc ISO-Compliant Enteral Syringe. J Pediatr Pharmacol Ther. 2023;28(3):255-261. doi: 10.5863/1551-6776-28.3.255. Epub 2023 Jun 2. PMID: 37303768; PMCID: PMC10249973
Laboratory testing shows that a mid-tolerance ENFit™ connector pair in a female-to-male orientation displaces an average volume of 0.148 ml of fluid, i.e., over-dosing of 0.148 ml (3)
(3) Extract of ISO 80369-3 - Annex A - Subpopulation within the enteral clinical application
On 12 October 2021, the FDA published a safety communication on LDT syringes relating to a risk of drug over-dosing with the ENFit® syringe with low-dose tip, based on a simulation carried out by ANDHEO, a specialised independent company, revealing over-dosing of 0.120 ml.
(4) Summary report - Study on the over-dosing risk of the "ENFit® Low Dose Tip" syringe during its use - March 2018, ANDHEO & Vygon.
- The connection device:
A poor connection between the feeding tube and the source of administration can lead to interruption of the infant's feeding, nutritional insufficiency or malabsorption. An incorrect connection to an IV or respiratory device can lead to the death of a preterm baby.
Numerous reports (4) describe the risks associated with errors in connecting enteral feeding tubes (such as tubes being connected to catheters or non-enteral tubes), sometimes with fatal consequences.
(4) Source: Maternal and Pediatric Nutrition – 2021 / 6:131. Safaa Abd EL Hamid Nasr EL Meneza (2021) Feeding Related Errors Jeopardize Safe Care in the Neonatal Intensive Care
Using a safe device is therefore vital for the safety of the preterm baby, as there is no risk of it being connected by mistake to other connections intended for other applications (intravenous, respiratory, etc.).
- Avoiding contamination:
Feeding tubes for newborns must never create a risk of bacterial contamination via the enteral route.
However, a study has shown that necrotising enterocolitis (NEC) develops in 5-10% of preterm babies in association with enteral feeding and bacterial colonisation (3).
We recommend using tubes with small connectors meant specifically for neonatology.
(3) Biofilm-based Healthcare-associated Infections - 2011 - Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Hum Dev. 2012 Mar;88 Suppl 1:S41-9. doi: 10.1016/j.earlhumdev.2011.12.027. Epub 2012 Jan 28. PMID: 22284985.
-
Do ENFit connectors™ ensure a suitable degree of cleanliness for newborns?
-
Several studies can answer this question:
• MEDSurg Nursing - 2020(1)
[...] ENFit® design [...]
The way Enfit® is designed poses a problem for cleaning the distal end of the tube. In fact, there is a hollow where residue from the enteral preparation or drugs are likely to accumulate and encourage proliferation of bacteria.
(1) Lyman, B., Rahe, K. L., Keeler, D., Sherman, A. K., & Abdelhadi, R. A. (2020). Randomized Controlled Trial Assessing the Effectiveness of Two Cleaning Regimens for ENFit® Connectors. Medsurg Nursing, 29(6), 401-406.• MEDSurg Nursing - 2020(2)
Comparing 2 cleaning protocols, protocol A / the more rigorous cleaning protocol and protocol B / the less rigorous cleaning protocol, the investigators found only 35 (31%) and 13 (16%) of the ENFit® connectors, respectively, to be free of residue after cleaning.
(2) Lyman, B., Rahe, K. L., Keeler, D., Sherman, A. K., & Abdelhadi, R. A. (2020). Randomized Controlled Trial Assessing the Effectiveness of Two Cleaning Regimens for ENFit® Connectors. Medsurg Nursing, 29(6), 401-406.
Preterm babies: best practices for enteral nutrition
While global recommendations encourage the use of a single connector for enteral nutrition in adult and neonatal patients, the French Society of Neonatology (SFN) is issuing a warning regarding the risk of inaccuracy of low doses with ENFit™ syringes.
- Using a safe and precise device
To guarantee the safety of preterm babies, we need to use appropriate, safe medical devices that provide a reliable, secure connection. As a reminder, ISO 80369-3 - 2016(1) emphasises that “in a 500g newborn infant, enteral drugs are often prescribed in volumes as small as 0.1 ml or even 0.01 ml”.
(1) Extract of ISO 80369-3 – Annex E - §E.5 Generic user needs - Using a suitable medical device
Preterm patients require extreme vigilance. Medical devices and connectors must be appropriate in size as far as possible. Specially designed for preterm babies, Nutrisafe2's compact design reduces the size, weight and dead space of the connector. Thanks to its reduced size, Nutrisafe2 reduces dead space by 70% compared to ENFit™/ISO 80369-3. Nutrisafe2 is a lightweight device, ideal for newborns, which reduces the risk of displacement or kinking of the tube. What’s more, the connector is completely smooth so as not to irritate the skin of the preterm patient in the event of contact. - Respecting protocols and training in the use of medical devices
Establishing standardised protocols and procedures for the administration, monitoring and maintenance of medical devices is essential to guarantee the health and safety of preterm babies. Healthcare professionals must be trained in the correct procedures for using enteral nutrition devices. Implementing cross-checking measures to minimise calculation, dosage and preparation errors can be a good way of reducing risks.

Enteral nutrition for preterm babies: the importance of using a dedicated medical device
While the French Society of Neonatology recommends the use of a safe system dedicated exclusively to enteral nutrition for newborns, we would like to draw attention to the risks associated with potential errors linked to the use of a single nutrition device for all patients. Ensuring safety, precision and the well-being of preterm babies means using a dedicated medical device, designed and adapted to their physiology and needs.